efficient sterile q tips Polypropylene handle factory price for test residues of previously manufactured products
◔ Cleanmo’s Total organic carbon (TOC) analysis is a fast and effective analytical technique for cleaning validation in pharmaceutical manufacturing.
◔ It has been designed to simplify sampling for cleaning validation in the pharmaceutical industry.
◔The swabs can also be used with Sievers, Tekmar Dohrman and Anatel A 2000 to measure TOC.
◔ The Swabs contains components for sampling 12 resp. 72 different areas in the production area.
◔ TOC analysis can be adapted to any drug compound or cleaning agent that contains carbon. The method is sensitive to the ppb range and is less time consuming than HPLC or UV/Vis. USP TOC methods are standard for Water for Injection and Purified Water2, and simple modifications of these methods can be used for cleaning validation.
◔ TOC of swab < 50 PPb
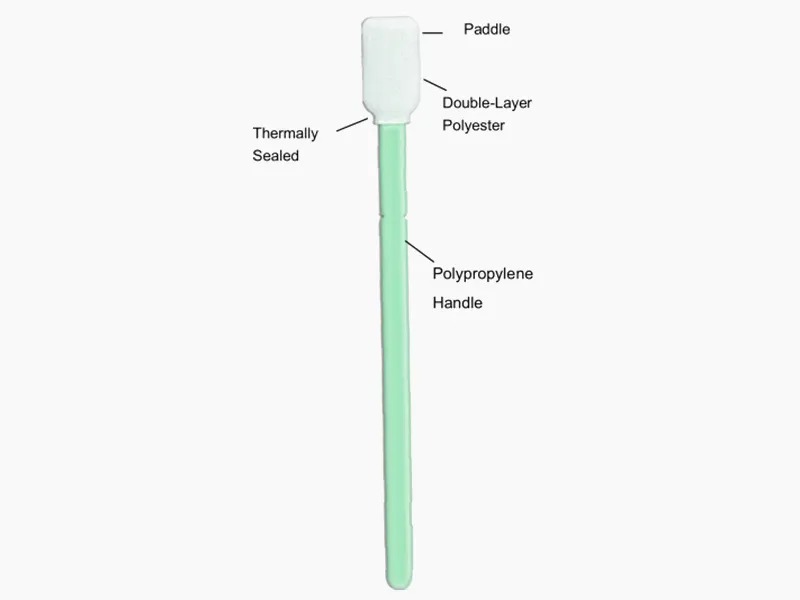
◔ Cleaning Validation Swabs
-Material: knitted polyester
-Head :Double layered
-Packing:Double packed
◔ Washed by EDI pure water
-Low particle generation
◔ Double layered head
-Good absorbent capacity
-Very good chemical resistance
◔ Handle
-Breakaway handle allows swabhead to be placed into vial with minimal handling and contamination
◔ Thermal bonded
-No contaminating adhesives or coating
◔ Autoclavable to ensure sampling under sterile conditions
◔ Laundered and packed under an ISO Class 5 Cleanroom
◔ Shelf life :2 years
❈ Technical Details
❈ Drawing and Size
| Part NO. | head material | head width | head thickness | head length | handle material | handle width | handle length | total length | packing |
| CM-PS713-TOC | Polyester | 12.3mm 0.484" | 3.3mm 0.130" | 23.0mm 0.906" | polypropylene | 5.8mm 0.228" | 100.0mm 3.937" | 123.0mm 4.843" | 100swabs/bag 50bags/ctn |
❈ Packing
Packing material:PO bag
Packing details:100pcs/bag,50pcs/bag,20pcs/bag,5000pcs/carton
Label on the packing can be designed as your requirement.
❈ Product Application
- TOC for the analysis of rinse water samples for cleaning validation determined trace levels of proteins, nucleic acids, amino acids, sugars.
- TOC test can be used to test for residues of previously manufactured products, cleaning detergents, chemicals, solvents, by-products,degradants,and microbial contaminants.
CONTACT US
TEL : (+86)755-29455975
E-MAIL : info@cleanmo.com
FAX:(+86)755-61605135
OFFICE ADD : Room 908, 9/F., Qinchengda Building, Xin'an Street, Bao'an District, Shenzhen city, Guangdong Province P.R.China